ACT & Parasite Clearance Review
Detailed summary of all published studies using an artemisinin regimen for uncomplicated Plasmodium falciparum malaria
The widespread deployment of Artemisinin Combination Therapies (ACTs) is widely acknowledged to be a key element in the substantial gains made by malaria control programs in many malaria endemic countries. However, the continued success of these malaria control programmes is under serious threat from the emergence of artemisinin-resistant strains of P. falciparum reported from the Thai-Cambodian border and more recently from the border areas of Thailand, Myanmar and Viet Nam (1, 2, 3, 4).
As advocated by the WHO, epidemiological surveillance of artemisinin resistance and delineation of the boundaries of spread are critical if appropriate public health measures are to be taken to delay the evolution of the resistance and eliminate pockets of resistant parasites when they are identified.
The current definition of artemisinin resistance depends upon quantitative microscopic measurement of delayed parasite clearance in the first few days following treatment with an ACT or artemisinin monotherapy. A variety of parameters have been developed for surveillance which vary in complexity as well as sensitivity. One approach advocates measuring the proportion of patients with detectable parasitaemia in a blood film taken on Day 3, after initial anti-malarial treatment. This simple approach can be useful since it is a parameter that is collected as part of the WHO guidelines for treatment efficacy, but a number of important factors may confound its interpretation, so additional options should be explored.
In the current literature review, the utility of applying a different marker of artemisinin resistance is assessed: a threshold of 10% of patients who remained parasitaemic on day 3 after treatment (5). The analysis included all available data on ACT treatment published between 2000-2011.
The WWARN team conducted a systematic search of the literature to identify all published studies using an artemisinin regimen for uncomplicated falciparum malaria published between January 2000 and December 2011. Data from these studies were extracted for analysis using a standardised questionnaire. You can download the full database of references and extracted data, along with various supplementary files (see Related Documents section). Finally, the group reviewed the trial methodology and the early parasitological response following treatment.
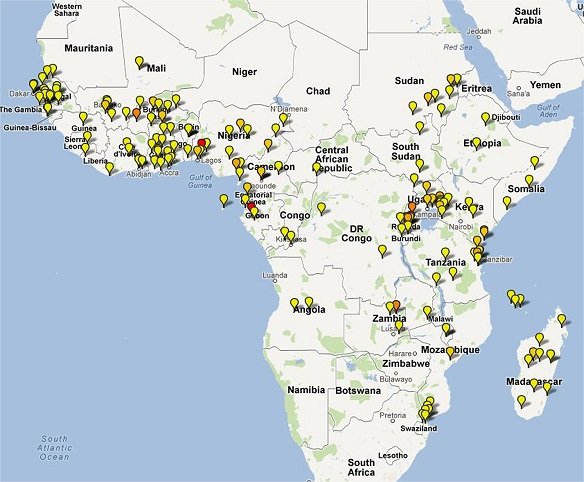
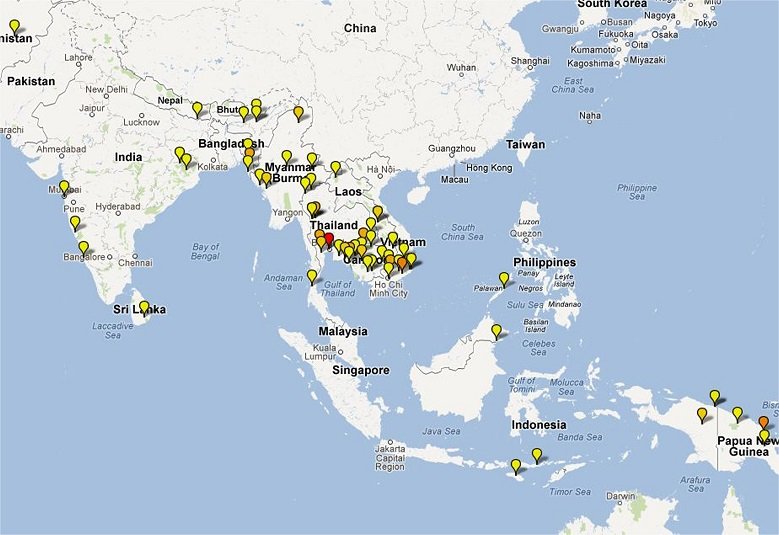
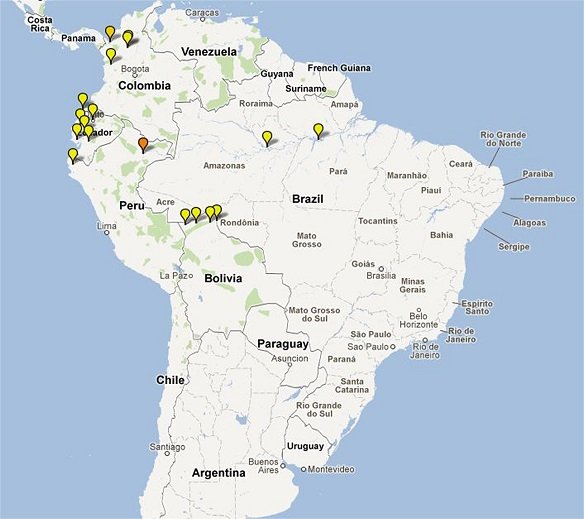
Clinical Trial Maps display where published trials took place. Each pin represents a single site where patients were recruited as part of an antimalarial clinical trial. Coloured icons denote number of studies at each site (yellow = 1; light orange = 2; dark orange = 3; red = >4).
Studies
In total 65,078 patients were enrolled into 213 clinical trials with 413 treatment arms containing either an artemisinin derivative alone (n=26) or in combination with a partner drug (n=387).
The median sample size per treatment arm was 110 (range 10-1,475), with most of the data derived from comparative drug trials (189, 89%), of which 177 (83%) were randomised trials. The remaining 24 (11%) studies were single-arm studies.
The most frequent treatment regimens reported were: artemether-lumefantrine (AL, in 96 treatment arms), artesunate-mefloquine (AS+MQ, N=75), artesunate-amodiaquine (AS+AQ, N=74), artesunate-sulphadoxine-pyrimethamine (AS+SP, N=43), and dihydroartemisinin-piperaquine (DHA+PIP, N=40). In 26 treatment arms, patients received monotherapy only, including either artesunate (N=17), dihydroartemisinin (N=1), artemisinin (N=3), beta-artemether (N=2) and arterolane (N=3) (as shown in the pie chart).
Where described, treatment was supervised completely in 362 (90%) and partially in 30 (7.5%) of the 402 studies.
The proportion of patients remaining parasitaemic at Day 1, Day 2 and Day 3 after treatment was documented in 115 (28%), 167 (40%) and 153 (37%) treatment arms, respectively.
- Excluding resistance studies in Cambodia, the median proportion of patients still parasitaemic was Day 1, 53.8% [range 3-95, IQR=30.5-69.2], Day 2, 6% [range 0-65.9, IQR=2-11.5] and Day 3, 0 [range 0-12.6, IQR=0-2].
- Comparing studies from 2000 to 2005 and 2006 to 2011, the median proportion of patients reported to remain parasitaemic at Day 3 decreased in Africa (1.2% vs 0%, p=0.007), but increased in Asia (0.4% vs 3.9%, p=0.076).
- In 95% of studies the proportion of patients with peripheral parasitaemia was less than 6% at Day 3.
These results highlight the normal distribution of early parasitological responses, and the influence of the heterogeneity in study design, host and parasite factors that confound a surveillance system based on Day 3 parasite positivity. Greater understanding of the factors influencing parasite clearance is crucial, but will require analysis of pooled data from individual patient records.
View the article: Das D, Price R, Bethell D et al. Early parasitological response following artemisinin-containing regimens: a critical review of the literature. Malaria Journal 2013; 12:125 doi:10.1186/1475-2875-12-125.