IDDO legal and regulatory documentation
IDDO maintains documentation to ensure compliance with the global data protection regulatory landscape, in particular the EU General Data Protection Regulation (GDPR), UK Data Protection Act 2018 and the UK GDPR. Documentation available on request: email us at info@iddo.org.
Key legal and regulatory documentation
The IDDO Terms of Submission sets out the legal terms and conditions for submitting your raw data to the IDDO data platform. This document sets out licensing and governance terms, your obligations as a data contributor and our obligations as the data processor, your choices about how access requests to your data are handled, important details of our data curation processes, and other important information.
Please do read the agreement carefully before submitting data to us and agreeing to the terms, together with our list of frequently asked questions. Email us at info@iddo.org for a copy of this document.
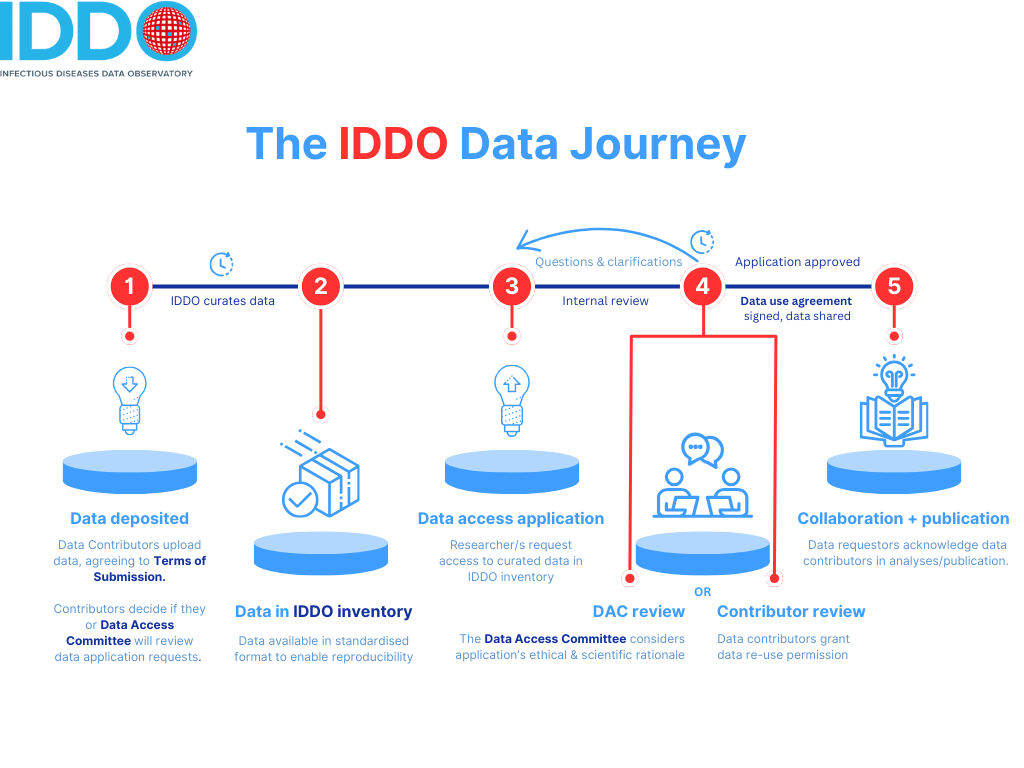
The IDDO Data Access Committee (DAC) is an independent committee chaired by TDR (the Special Programme for Research and Training in Tropical Disease), on behalf of the World Health Organization. The DAC provides an indepenent decision-making mechanism to evaluate and decide on data access applications for IDDO-curated data.
Key documents regulating DAC function include:
- the DAC Terms of Reference. These set out the purpose, membership, eligibility, duties and other terms and conditions for the IDDO DAC.
- The IDDO Conflict of Interest Policy covers all members of the IDDO DAC, the IDDO Scientific Advisory and other governance committees, the IDDO secretariat, and any individuals working in an advisory capacity for IDDO. The policy sets out definitions of a conflict of interest, dispute resolution, and other matters relating to potential conflicts of interest.
- the IDDO Platform Data Access Guidelines set out the basis on which the IDDO DAC makes decisions on applications for data access. It sets out principles of access to data that the Committee works within, the process for approval and data access, and the points that the Committee considers when making a decision on applications.
See our list of frequently asked questions for more information, and make a request for data for the particular disease data you are interested in via our research theme pages. Get in touch with us at info@iddo.org if you have any further questions or would like a copy of any of these documents.
This document sets out the basis on which the IDDO Data Access Committee makes decisions on applications for data access. It sets out principles of access to data that the Committee works within, the process for approval and data access and the points that the Committee considers when making a decision on applications.
If you are considering submitting a data access application or working within the Data Access Committee, email us at info@iddo.org to request this document. Please also see our list of frequently asked questions.